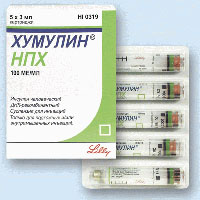
Humulin NPH (Insulin)
- EU domestic delivery
- Payment methods: Sigue, RIA,
 , Bank transfer, Bitcoin BTC.
, Bank transfer, Bitcoin BTC. - Shipping methods: Airmail, EMS
- Order through website or email
- Seizure reshipment policy
- Product guarantee
| Company: | Lilly (France) |
| Usage: | peptides |
|
5 Cartriges x 3ml. [100 IU/ml.] Total 1500IU Price:65$ |
|
| Shipping Location: | EU |
| brands: | TOP PHARMA GRADE PRODUCTS |
| type (oral/injectable): | PEPTIDES |
| type (oral/injectable): | EU domestic delivery |
| compound: | Insulin |
| Antidiabetic Agent | |
| Rate: | Not rated |
| HUMULIN® |
| Lilly |
| Insulin, Human Biosynthetic |
| Antidiabetic Agent |
Action And Clinical Pharmacology: Humulin is a polypeptide hormone consisting of a 21 amino acid A-chain and a 30 amino acid B-chain linked by two disulfide bonds.
Humulin is found to be chemically, physically, biologically and immunologically equivalent to pancreatic human insulin which differs slightly from porcine or bovine insulin in amino acid composition.
Studies indicate immunogenicity problems with biosynthetic human insulin (BHI) produced by recombinant DNA technology are less likely than with insulin derived from animal origin. Biosynthetic human insulin is devoid of all protein contaminants of pancreatic origin normally present in trace amounts in all insulins of pancreatic origin. The purification procedures used in the manufacture of biosynthetic human insulin result in a product which contains an insufficient quantity of E. coli polypeptides to be antigenic in deliberately sensitized animals. No antibodies to E. coli polypeptides have been detected in specifically designed radioimmunoassay methods examining patient serum samples.
The administration of suitable doses of insulin to patients with diabetes mellitus, along with controlled diet and exercise, temporarily restores their ability to metabolize carbohydrates, fats and proteins; to store glycogen in the liver; and to convert glucose to fat. When given in suitable doses at regular intervals to a patient with diabetes mellitus, the blood sugar is maintained within a reasonable range, the urine remains relatively free of sugar and ketone bodies, and diabetic acidosis and coma are prevented.
Insulin preparations differ in onset, peak and duration of action. The addition of protamine to insulin, in the presence of zinc, produces a stable complex with less intense and more prolonged action, due to its slow dissolution.
Humulin-R, Regular, Insulin Injection, Human Biosynthetic, is a rapidly acting insulin with a relatively short duration of activity (6 to 8 hours).
Humulin-N, NPH, Insulin Isophane, Human Biosynthetic, is an intermediate-acting insulin with a slower onset of action than Regular insulin and a longer duration of activity of up to 24 hours.
Humulin-L, Lente, Insulin Zinc Suspension Medium, Human Biosynthetic, is an intermediate-acting insulin with a slower onset of action than Regular insulin and a longer duration of activity of up to 24 hours.
Humulin-U, Ultralente, Insulin Zinc Suspension Prolonged, Human Biosynthetic, is a long-acting insulin with a slower onset of action than Regular insulin and a longer duration of activity (of at least 24 hours or more).
Humulin Mixtures (10/90, 20/80, 30/70, 40/60 and 50/50 Insulin Injection, Human Biosynthetic and Insulin Isophane, Human Biosynthetic) are intermediate-acting insulins with a more rapid onset of action than NPH alone and a duration of activity of up to 24 hours.
Humulin-N, Humulin-L or Humulin-U may be mixed with Humulin-R to meet individual metabolic requirements of the patient as determined by the physician.
Indications And Clinical Uses: For the treatment of insulin-requiring diabetic patients.
Humulin-R only should be used for the treatment of emergencies such as diabetic coma and pre-coma, in diabetics undergoing surgery, but not Humulin-N, Humulin-L, Humulin-U or Humulin Mixtures.
In switching patients from animal source insulins to Humulin, it is possible that the patients will require a change in dosage; the adjustment may be made with the first dose or over a period of several weeks. Any change of insulin should be made cautiously and only under medical supervision.
Changes in refinement, purity, strength, brand, type and/or method of manufacture (recombinant DNA versus animal source insulin) may result in the need of a change in dosage.
Contra-Indications: Hypoglycemia (see Hypoglycemia in Overdose).
Humulin-N, Humulin Mixtures, Humulin-L and Humulin-U should not be given i.v. or used for treatment of diabetic coma.
Manufacturers` Warnings In Clinical States: A few patients who experienced hypoglycemic reactions after being transferred to Humulin have reported that these early warning symptoms were less pronounced than they were with animal-source insulin.
Under no circumstances should any Humulin Mixture be given i.v.
Do not use the Humulin-N, Humulin-L, Humulin-U or Humulin Mixtures if you see lumps that float or that stick to the sides of the vial, or if the contents of the vial are clear and remain clear after the bottle is shaken or rotated. Note: The contents of the vial of Humulin-R should be clear. Do not use if cloudy.
Precautions: Visual disturbances in uncontrolled diabetes due to refractive changes are reversed during the early phase of effective management. However, since alteration in osmotic equilibrium between the lens and ocular fluids may not stabilize for a few weeks after initiating therapy, it is wise to postpone prescribing new corrective lenses for 3 to 6 weeks.
Insulin requirements may be increased during illness or emotional disturbances or if the patient is receiving concurrent administration of drugs with hyperglycemic activity, e.g. oral contraceptives, corticosteroids or thyroid replacement therapy.
Insulin requirements may be reduced in the presence of renal or hepatic impairment or if the patient is receiving concurrent administration of drugs with hypoglycemic activity, e.g. monoamine oxidase inhibitors and beta-adrenergic blockers.
The number and size of daily doses and the time of administration, as well as diet and exercise, are problems that require direct and continuous medical supervision. Usually, the most satisfactory injection time is before breakfast.
Prompt recognition and appropriate management of the allergic complications of insulin therapy are essential for the safe and effective control of diabetes mellitus.
Transferring from Other Insulins: A small number of patients transferring from insulins of animal source to insulins of recombinant DNA origin may require a reduced dosage, especially if they are tightly controlled and bordering on hypoglycemia. The dosage reduction may occur with the first dose or over a period of several weeks. There is a risk of hypoglycemia if the insulin requirement is decreased, and both the physician and the patient should be aware of this possibility. The risk can be considered to be minimal if the daily dose is less than 40 units.
Pregnancy: It is essential to maintain good control of the insulin-diabetic patient throughout pregnancy. Insulin requirements usually decrease during the first trimester and increase during the second and third trimesters.
Lactation: Diabetic patients who are nursing may require adjustments in insulin dose and/or diet.
Drug Interactions: Hormones that tend to counteract the hypoglycemic effects of insulin include growth hormone, corticotropin, glucocorticoids, thyroid hormone and glucagon. Epinephrine not only inhibits the secretion of insulin, but also stimulates glycogen breakdown to glucose. Thus, the presence of such diseases as acromegaly, Cushing`s syndrome, hyperthyroidism, and pheochromocytoma complicate the control of diabetes.
The hypoglycemic action of insulin may also be antagonized by phenytoin. Insulin`s hypoglycemic action can be increased in some patients by concomitant administration of anabolic steroids, MAO inhibitors, guanethidine, alcohol, propranolol (masking effect) or other drugs affecting beta-adrenergic receptors, or by daily doses of 1.5 to 6 g of salicylates.
Insulin requirements can be increased, decreased, or unchanged in patients receiving diuretics. Concomitant administration of oral contraceptives can cause a decrease in glucose tolerance in diabetic women possibly resulting in increased daily insulin requirements.
Adverse Reactions: Since Humulin has been available worldwide, reports of local and systemic allergic reactions in patients receiving it have been received. As with all insulins, local inflammatory responses may result from improper cleansing of the skin, contamination of the injection site with alcohol, use of an antiseptic containing impurities or accidental intracutaneous rather than s.c. injection. Local reactions that result in skin sensitivity phenomena usually subside spontaneously.
Insulin lipohypertrophy has been reported with Humulin. This complication has been ascribed to the local pharmacologic effects of the s.c. injection of insulin. A few cases of lipoatrophy and serum sickness have also been reported.
Symptoms And Treatment Of Overdose: Hypoglycemia: Cause: Hypoglycemia (low blood glucose, also called "insulin reaction") can occur if the patient takes too much insulin, misses meals, exercises or works too hard just before a meal, or has an infection or becomes ill (especially with diarrhea or vomiting) or if his/her body`s need for insulin changes for other reasons.Symptoms: Hypoglycemia may occur in any patient receiving insulin and is most commonly manifested by hunger, nervousness, warmth and sweating, and palpitations. Patients also may experience headache, confusion, drowsiness, fatigue, anxiety, blurred vision, diplopia, or numbness of the lips, nose or fingers. The clinical manifestations of hypoglycemia can be masked by the concomitant administration of propranolol or other beta-adrenergic blockers.
Symptoms are likely to appear anytime when the blood sugar concentration falls below 2.2 mmol/L (40 mg/100 mL) but may occur with a sudden drop in blood glucose even when the value remains above 2.2 mmol/L (40 mg/100 mL).
Treatment: If a patient is unable to take soluble carbohydrate or fruit juice orally, hypoglycemia is treated with 10 to 20 g of dextrose i.v. or glucagon may be given s.c. or i.m.
Dosage And Administration: The dosage should be determined by the physician, according to the requirements of the patient.
New Patients: Patients receiving insulin for the first time can be started on Humulin in the same manner as they would be on animal-source insulin.
Patients should be monitored closely during the adjustment period.
Transfer Patients: When transferring patients from animal-source insulin to Humulin, use the same dose and dosage schedule.
Some patients transferring to Humulin will require a change in dosage from that used with animal-source insulin. If an adjustment is needed, it may be made with the first dose or over a period of several weeks.
Changes in total daily dosage, the number of injections per day, and/or timing of injections may be necessary to achieve maximum glycemic control.
When a patient on high doses of animal insulin is switched to Humulin, it may be appropriate to reduce the starting dosage and monitor the patient carefully.
Patients who have systemic allergy to pork or beef insulin may also react to human insulin. In such patients, appropriate procedures (intradermal testing and, if necessary, desensitization) should be undertaken before therapeutic doses of human insulin are administered.
A few patients who experienced hypoglycemic reactions after being transferred to Humulin have reported that the early warning symptoms, i.e., nervousness, sweating and palpitations, were less pronounced than they were with animal-source insulin.
Formulations of Humulin appear to produce a slightly faster onset and slightly shorter duration of action than the corresponding forms of animal-source insulins.
Humulin-R is a clear, colorless solution. It may be administered by s.c., i.m. or i.v. injection.
Humulin-N, Humulin Mixtures, Humulin-L and Humulin-U are suspensions. They should be administered by s.c. injection only.
S.C. administration, preferably by the patient, should be in the upper arms, thighs, buttocks or abdomen. Injection sites should be rotated so that the same site is not used more than approximately once a month.
Care should be taken to ensure that a blood vessel has not been entered. The injection site should not be massaged.
Mixing Instructions: The rapid action of Humulin-R is preserved when mixed with Humulin-N; independent of the time lag between mixing and administration, and independent of the proportion of regular insulin incorporated in the mixture.
Humulin-L and Humulin-R bind immediately after mixing resulting in a delay in the onset of the regular insulin action, reduction in peak activity magnitude, and prolonged total duration of activity.
Mixing Humulin-R and Humulin-U results in reduction of the quick-acting effect of regular insulin.
The effects of mixing Humulin with animal-source insulins have not been studied. This practice is not recommended.
Stability and Storage: Insulin should be stored in a cold place (2 to 10°C), preferably in a refrigerator, but not in a freezer. Do not let it freeze or leave it in direct sunlight. Expiration dates are stated on the labels.
When in use, vials and cartridges may be kept at room temperatue for up to 28 days.
Availability And Storage: Humulin-R: Each mL contains 100 units of Regular insulin. Nonmedicinal ingredients: glycerol and m-cresol. May contain: dimethicone, hydrochloric acid and sodium hydroxide. Cartridges of 1.5 and 3 mL, boxes of 5. Vials of 10 mL.
Humulin-N: Each mL contains 100 units of NPH insulin. Nonmedicinal ingredients: dibasic sodium phosphate, glycerol, m-cresol, phenol, protamine sulfate and zinc. May contain: dimethicone, hydrochloric acid and sodium hydroxide. Cartridges of 1.5 and 3 mL, boxes of 5. Vials of 10 mL.
Humulin-L: Each mL contains 100 units of Lente insulin. Nonmedicinal ingredients: methyl paraben, sodium acetate, sodium chloride and zinc. May contain dimethicone, hydrochloric acid and sodium hydroxide. Vials of 10 mL.
Humulin-U: Each mL contains 100 units of Ultralente insulin. Nonmedicinal ingredients: methyl paraben, sodium acetate, sodium chloride and zinc. May contain: dimethicone, hydrochloric acid and sodium hydroxide. Vials of 10 mL.
Humulin 10/90: Each mL contains 10 units of Regular insulin and 90 units of NPH insulin. Nonmedicinal ingredients: dibasic sodium phosphate, glycerol, m-cresol, phenol, protamine sulfate and zinc. May contain: dimethicone, hydrochloric acid and sodium hydroxide. Cartridges of 1.5 and 3 mL, boxes of 5. Vials of 10 mL.
Humulin 20/80: Each mL contains 20 units of Regular insulin and 80 units of NPH insulin. Nonmedicinal ingredients: dibasic sodium phosphate, glycerol, m-cresol, phenol, protamine sulfate and zinc. May contain: dimethicone, hydrochloric acid and sodium hydroxide. Cartridges of 1.5 and 3 mL, boxes of 5. Vials of 10 mL.
Humulin 30/70: Each mL contains 30 units Regular insulin and 70 units of NPH insulin. Nonmedicinal ingredients: dibasic sodium phosphate, glycerol, m-cresol, phenol, protamine sulfate and zinc. May contain: dimethicone, hydrochloric acid and sodium hydroxide. Cartridges of 1.5 and 3 mL, boxes of 5. Vials of 10 mL.
Humulin 40/60: Each mL contains 40 units of Regular insulin and 60 units of NPH insulin. Nonmedicinal ingredients: dibasic sodium phosphate, glycerol, m-cresol, phenol, protamine sulfate and zinc. May contain: dimethicone, hydrochloric acid and sodium hydroxide. Cartridges of 1.5 and 3 mL, boxes of 5. Vials of 10 mL.
Humulin 50/50: Each mL contains 50 units of Regular insulin and 50 units of NPH insulin. Nonmedicinal ingredients: dibasic sodium phosphate, glycerol, m-cresol, phenol, protamine sulfate and zinc. May contain: dimethicone, hydrochloric acid and sodium hydroxide. Cartridges of 1.5 and 3 mL, boxes of 5. Vials of 10 mL.
Humulin cartridges are designed for use with the B-D Pen, B-D Pen Ultra Cartridge System, or future Lilly injector systems.
Read more...

![Buy HGH (Human Growth Hormone) [Somatropin] [Kigtropin] - Kigtropin (China)](/get_image/kigtropin.jpg)
![Buy Cabaser [Dostinex] (Cabergoline) - Pharmacia & Upjohn (Italy)](/get_image/cabaser.jpg)
![Buy Saizen (HGH, Human Growth Hormone) [Somatropin] - Merck Serono](/get_image/saizen_click_easy.jpg)

![Buy Clomid [Clomiphene Citrate] (Clomiphene Citrate) - Anfarm Hellas (Greece)](/get_image/clomiphene48.jpg)
![Buy SP Nandrolone (Deca-Durabolin) [Nandrolone Decanoate] - SP laboratories (Moldova)](/get_image/spdeca.jpg)